What are the Documents Required for a Drug License?
In India, if a business or company works in the health care sector such as cosmetics, medicines, or drugs, they shall get the Drug License, which is issued by State/Central Drug Standard Control Organization. Without this license, nobody can start any pharmacy business in India; doing so is a cognizable offense. Drugs are specially used in medicines which cure almost any diseases. The preparation and usage of an excessive amount of drugs in medicines are restrictive, and all the rules are set for using the right amount of drugs used in medication so that they didn't harm the body by its overdose. If the amount of drugs is high in medicine, it will severely affect the person who consumes it.
What are the various kinds of Drug Licenses?
The license is granted on the basis of the type of business. Following are the different types of licenses:

Requirements to obtain Drug License in India
Following are the essential requirements that you must know before applying for a license in India:
Minimum 10 square meters area is required for setting up a medical shop or retail pharmacy. If they are operating the wholesale business, then the minimum area required for operating the business shall be 15 square meters.
The implementation of cold storage or refrigerators for the storage of medicines and vaccines as they will be stored in cold places, Medical shops or any retail pharmacy must have a technical and qualified staff with thorough knowledge of the same.
Essential documents for obtaining Drug License
Below is the list of all the vital documents required for registration in India:
- Submit a duly signed Application form, which is already filled.
- Copy of challan.
- Identity proof such as PAN card, Driving license, Aadhar card, passport, Voter Id in case an applicant is a proprietor.
- Submit an Identity proof of every partner along with the partnership deed (in case of the applicant is a partnership firm).
- Copy of AOA (Articles of Association) and MOA (Memorandum of Association) in case of Public Limited / One Person Company / Private Limited.
- Submit an Appointment letter along with bio-data.
- Submit a one-year Experience Certificate.
- Submit a copy of the rental agreement if the office is rented.
- Invoice of refrigerators in use.
- Registration Certificate with the Delhi Pharmacy Council.
- Obtain degree certificates, provisional certificates, or mark sheets as educational proof.
Documents submitted by Registered Pharmacist:
- Submit a Certificate of Final Degree along with the mark sheets.
- Delhi Pharmacy Council Registration.
- Submit the Appointment Letter and a copy of Bio-data.
Documents submitted by Competent Person:
- Submit a Certificate of Final Degree along with the mark sheets.
- Submit the Appointment Letter and a copy of Bio-data.
- Minimum one-year Experience Certificate.
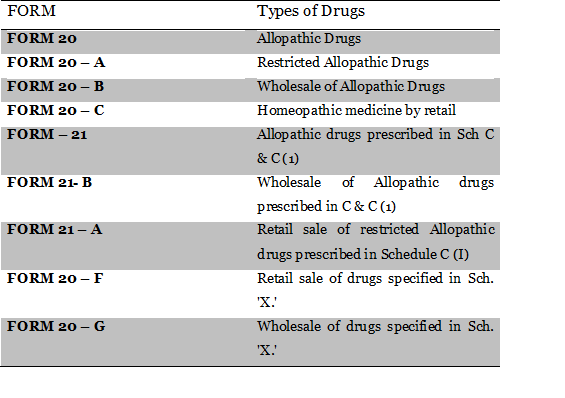
Different types of forms of License for sale and wholesale of drugs
Procedure to Obtain Drug License in India
Normally, the process takes as long as 30-60 days; the following are the steps to get a License in India:
Step 1: First, the applicant must apply online for the license, where the applicant must have a valid contact number and email id.
Step 2: All the necessary documents must be scanned in a 100PPI (Pixel Per Inch) size and upload the documents & the online application form for the license along with the application fees.
Step 3: Once the online application process is done, an examiner or authorized officer will visit the premises and verify all the essential documents' validity.
Step 4: Once your application review is done, the examiner or inspector will approve the application, and then the authority will issue the license.
Conclusion
The requirement of license in India is very important because it promotes public health, and it regulates the sale, distribution, and drugs import. Drug license ensures that the organization is following all the rules and regulations set by the State/Central Drug Standard Control Organization.
